-
Enantioseparation of baclofen with highly sulfated β-cyclodextrin by capillary electrophoresis with laser-induced fluorescence detection
G. Kavran Belin, S. Rudaz and J.-L. Veuthey
Journal of Separation Science, 28 (16) (2005), p2187-2192


DOI:10.1002/jssc.200500100 | unige:3629 | Abstract | Article PDF
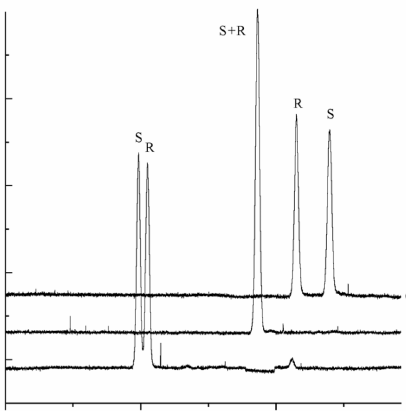
The enantioseparation of baclofen (4-amino-3-p-chlorophenylbutyric acid) was achieved by CE-LIF with highly sulfated β-CD (HS-β-CD) as chiral selector. Naphthalene-2,3-dicarboxaldehyde was used for the derivatization of nonfluorescent baclofen. HS-β-CD (2%) containing 50 mM borate buffer at pH 9.5 was chosen as the optimal running electrolyte and applied to the analysis of baclofen enantiomers in human plasma. The linearity of calibration curves (R 2 ≥ 0.998) for R-(-) and S-(+)-baclofen was in the 0.1-2.0 μM concentration range. After a simple ACN-protein precipitation, the LOD of baclofen in plasma sample was found as low as 50 nM.